by RESA, mpt
Abstract
Canine osteoarthritis (OA) is a prevalent, chronic, and degenerative joint disease that significantly impairs the quality of life and welfare of affected animals. Management of the associated chronic pain presents a considerable challenge for veterinary practitioners and pet owners, leading to the exploration of a wide spectrum of therapeutic options. This review conducts a comparative analysis of two distinctly different approaches to managing canine OA pain: monoclonal antibody (mAb) therapy, a novel, evidence-based biologic intervention, and homeopathic treatments, a form of complementary and alternative medicine. The analysis critically evaluates the underlying principles, mechanisms of action, and the strength of the clinical evidence base for each modality. Monoclonal antibody therapy, exemplified by nerve growth factor (NGF) antagonists, operates on a well-defined, biologically plausible mechanism to provide targeted analgesia. Its efficacy and safety are supported by a substantial body of evidence from rigorous, placebo-controlled clinical trials. In stark contrast, homeopathic principles, such as “like cures like” and extreme dilution, are inconsistent with established principles of pharmacology and chemistry. A critical review of the literature reveals that clinical studies on homeopathy for pain management are scarce, often methodologically flawed, and fail to demonstrate efficacy beyond a placebo effect. This paper argues that while mAb therapy represents a significant and scientifically validated advancement in the management of canine OA, homeopathic treatments lack a credible scientific basis and demonstrable clinical efficacy. The discussion emphasizes the importance of an evidence-based framework in veterinary practice, the ethical considerations of treatment selection, and the critical role of client communication in navigating treatment decisions to ensure optimal animal welfare.
1. Introduction
Canine osteoarthritis (OA) stands as one of the most common causes of chronic pain and disability in the domestic dog population. This progressive, degenerative joint disease culminates in the irreversible loss of articular cartilage, subchondral bone remodeling, and persistent inflammation, leading to pain, lameness, and a substantial reduction in mobility. The resultant decline in physical activity and behavioral changes profoundly impacts the human-animal bond and represents a significant animal welfare concern that veterinarians and pet owners grapple with daily. Management of this condition is palliative, focusing on alleviating pain, improving joint function, and slowing disease progression to maintain an acceptable quality of life for the patient.
The therapeutic landscape for canine OA is broad and continually evolving, encompassing a multimodal approach that typically includes weight management, physical rehabilitation, and pharmaceutical interventions. For decades, non-steroidal anti-inflammatory drugs (NSAIDs) have been the cornerstone of medical management. However, the requirement for long-term administration in a chronic disease raises concerns regarding potential adverse effects, creating an impetus for the development of safer and more targeted therapies. This search for effective and well-tolerated treatments has led veterinary medicine down two divergent paths.
One path follows the trajectory of modern biomedical innovation, culminating in the development of targeted biological therapies. Monoclonal antibody (mAb) therapy, specifically targeting nerve growth factor (NGF), represents a paradigm shift in veterinary analgesia. These therapies are the product of extensive research into the molecular drivers of osteoarthritic pain and have undergone rigorous regulatory scrutiny, supported by large-scale, randomized controlled clinical trials to establish their efficacy and safety. They epitomize the principles of evidence-based veterinary medicine, offering a precise mechanism of action aimed at a specific pathological target.
The other path ventures into the realm of complementary and alternative medicine (CAM), where homeopathy remains a popular choice among pet owners seeking “natural” or holistic options for chronic conditions like arthritis . Homeopathy operates on a set of principles fundamentally different from conventional pharmacology, proposing that highly diluted substances can stimulate a self-healing response. Despite its long history and dedicated following, homeopathy exists in a space of significant scientific controversy, with its proposed mechanisms challenging fundamental laws of physics and chemistry.
This paper provides a comprehensive, evidence-based comparative analysis of these two profoundly different therapeutic modalities for the management of canine OA. By critically examining the pathophysiology of the disease, the proposed mechanisms of action for each treatment, and the quality of the supporting scientific evidence, this review aims to construct a clear, evidence-based hierarchy for clinical decision-making. It will explore the vast chasm between the targeted biological intervention of mAb therapy and the philosophical underpinnings of homeopathy, evaluating the strength of their respective evidence bases—from robust clinical trials to anecdotal reports. Ultimately, this analysis seeks to equip veterinary professionals with the critical insights needed to navigate complex treatment conversations with pet owners, ensuring that therapeutic choices are guided by scientific integrity and a steadfast commitment to animal welfare.
2. The Pathophysiology and Management Landscape of Canine Osteoarthritis
Canine osteoarthritis is not merely a consequence of aging or simple “wear and tear” on joints but is now understood as a complex and dynamic disease of the entire synovial joint organ. Its development involves a vicious cycle of biomechanical and biochemical events that progressively degrade joint structures and perpetuate a state of chronic pain and inflammation. A thorough understanding of this pathophysiology is essential for evaluating the rationale and potential efficacy of any therapeutic intervention.
2.1. The Molecular and Biomechanical Basis of Canine OA
The pathogenesis of canine OA is multifactorial, involving an intricate interplay between mechanical forces and biological responses within the joint. In dogs, OA is most often secondary to an underlying developmental orthopedic disease, such as hip or elbow dysplasia, or an acquired condition like a cranial cruciate ligament (CrCL) rupture or joint trauma . These primary conditions result in joint instability and incongruity, which alter the normal biomechanical loading patterns across the articular surfaces . This abnormal mechanical stress is the critical initiating factor, triggering a cascade of cellular and molecular changes within the joint.
From a biomechanical perspective, abnormal loading leads to microdamage in the articular cartilage and changes in the underlying subchondral bone, which may become stiffer and less able to absorb shock . This mechanical failure initiates a maladaptive biological response from the chondrocytes, the cells responsible for maintaining the cartilage matrix. These stressed cells begin to produce a range of catabolic and pro-inflammatory molecules. The ensuing pathological process is characterized by a complex inflammatory and biomechanical chain reaction .
At the molecular level, this reaction involves the production and release of numerous inflammatory mediators, including cytokines (e.g., interleukin-1, tumor necrosis factor-alpha), prostaglandins, and catabolic enzymes such as matrix metalloproteinases (MMPs) . These mediators are produced by multiple joint tissues, including the synovium, cartilage, and bone, and they create a pro-inflammatory microenvironment within the joint . This inflammatory milieu is central to the progression of OA; it drives the degradation of the extracellular matrix of the cartilage, primarily composed of collagen and proteoglycans, leading to cartilage thinning, fibrillation, and eventual erosion down to the subchondral bone. Furthermore, these mediators can move from the primary joint pathology to affect surrounding periarticular tissues, contributing to the global pain state . The failure of ligaments, such as the CrCL, not only causes instability but also contributes directly to the biomechanical progression of OA and meniscal pathology . This self-perpetuating cycle, where mechanical damage incites an inflammatory response that in turn causes further tissue degradation and functional impairment, defines the progressive nature of the disease.
2.2. Clinical Manifestations and Impact on Animal Welfare
The underlying pathological changes of OA manifest clinically as a constellation of signs primarily related to chronic pain. Pet owners may observe overt signs such as lameness, a stiff or stilted gait, and difficulty rising from a resting position. Other common indicators include reluctance to engage in normal activities like jumping onto furniture, climbing stairs, or playing. The pain is often exacerbated by cold, damp weather or strenuous activity and may appear to improve with mild exercise.
Beyond these classic orthopedic signs, chronic OA pain frequently leads to significant behavioral changes. Dogs may become more irritable, withdrawn, or less interactive with family members. They might exhibit signs of anxiety or aggression when painful areas are touched. These subtle shifts in behavior can be easily misinterpreted or attributed to “old age,” causing the severity of the animal’s suffering to be underestimated. This chronic pain state is a profound welfare issue, leading to a progressive deterioration in the animal’s overall quality of life.
Diagnosis of canine OA relies on a combination of a thorough patient history provided by the owner, a comprehensive physical and orthopedic examination by a veterinarian, and radiographic imaging. While radiographs are essential for confirming the presence of characteristic changes—such as osteophyte formation, subchondral sclerosis, and joint space narrowing—the severity of radiographic findings does not always correlate directly with the degree of pain or functional impairment experienced by the patient. Therefore, clinical assessment, including gait analysis and validated owner-completed questionnaires, plays a crucial role in evaluating the individual patient’s pain level and response to treatment. The management goal is thus centered on breaking the pain cycle to improve function and restore a positive quality of life.
2.3. Conventional Pharmaceutical Interventions and Their Limitations
The management of canine OA is inherently multimodal, aiming to address the various facets of the disease through a combination of strategies. While non-pharmaceutical interventions such as weight optimization, controlled low-impact exercise, and physical rehabilitation are fundamental to any treatment plan, pharmaceutical intervention is almost always necessary to effectively control the associated pain and inflammation.
For many years, the cornerstone of medical management for canine OA has been the use of non-steroidal anti-inflammatory drugs (NSAIDs). These drugs exert their therapeutic effect primarily by inhibiting the cyclooxygenase (COX) enzymes, thereby reducing the production of prostaglandins that mediate pain and inflammation. Multiple formulations of veterinary-specific NSAIDs are available and have demonstrated efficacy in alleviating the clinical signs of OA. They remain a vital tool for managing acute flare-ups and providing foundational analgesia for many patients.
However, the chronic nature of OA necessitates long-term, often lifelong, administration of analgesics, which brings the limitations of NSAIDs into sharp focus. Their mechanism of action is not entirely specific to inflammatory pathways; inhibition of COX enzymes can also interfere with homeostatic functions in the gastrointestinal tract, kidneys, and liver. Consequently, long-term use of NSAIDs carries a risk of adverse effects, including gastrointestinal ulceration, nephrotoxicity, and hepatotoxicity. While newer NSAIDs with greater selectivity for the COX-2 isoenzyme have improved safety profiles compared to older, non-selective compounds, the risk is not eliminated. This necessitates careful patient selection and regular monitoring, and some patients with pre-existing comorbidities may not be suitable candidates for NSAID therapy at all.
This therapeutic gap—the need for safe, effective, long-term analgesia with a more targeted mechanism of action and fewer systemic side effects—has been a major driver of pharmaceutical research. The limitations of conventional therapies highlight the demand for novel interventions that can provide sustained pain relief while minimizing risks to the patient. It is within this context that innovative approaches like monoclonal antibody therapy have emerged. The reliance of veterinary professionals on a hierarchy of evidence, often adapted from human medicine, underscores the importance of critically evaluating both established and emerging therapies based on robust scientific data . This unmet clinical need also helps to explain why some pet owners, concerned about the long-term use of conventional drugs, turn to alternative options like homeopathy in search of a perceived safer solution for their companions’ chronic pain.
3. Monoclonal Antibody Therapy: A Targeted Molecular Approach
Monoclonal antibody (mAb) therapy represents a paradigm shift in the management of canine osteoarthritis (OA), moving away from broad-spectrum anti-inflammatory agents toward highly specific, targeted biological interventions. This approach is rooted in a deep understanding of the molecular pathophysiology of OA-associated pain, aiming to neutralize key signaling molecules with high precision. The development and regulatory approval of these therapies, such as bedinvetmab, are underpinned by extensive preclinical and clinical research, establishing a new standard for evidence-based pain management in veterinary medicine . This section will explore the mechanism of action, clinical evidence, and real-world application of mAb therapy for canine OA, highlighting its scientific foundation and therapeutic impact.
3.1. Mechanism of Action: Neutralizing Key Pain and Inflammation Mediators
The therapeutic strategy of monoclonal antibodies in canine OA is centered on the targeted neutralization of Nerve Growth Factor (NGF), a neurotrophin identified as a pivotal mediator of chronic pain. While historically known for its role in neuronal development and survival, a substantial body of preclinical and clinical evidence has now firmly established NGF’s function in the sensitization of the peripheral and central nervous systems in chronic pain states . In the context of an osteoarthritic joint, inflamed and damaged tissues such as the synovium, chondrocytes, and subchondral bone produce elevated levels of NGF. This surplus of NGF binds to its high-affinity receptor, Tropomyosin receptor kinase A (TrkA), which is expressed on the surface of nociceptive (pain-sensing) neurons.
The binding of NGF to TrkA receptors initiates a cascade of downstream signaling events that orchestrate the pain experience associated with OA. This includes upregulating the expression of other pain-related proteins like substance P and calcitonin gene-related peptide (CGRP), as well as ion channels such as TRPV1, which lowers the activation threshold of nociceptors. The result is peripheral sensitization, a state where nerve endings become hyper-responsive to both noxious and non-noxious stimuli, leading to primary hyperalgesia (exaggerated pain from a painful stimulus) and allodynia (pain from a normally non-painful stimulus). Furthermore, the continuous afferent barrage from sensitized peripheral nerves can induce changes in the dorsal horn of the spinal cord, leading to central sensitization, which amplifies and prolongs the pain state.
Monoclonal antibody therapy, specifically with a caninized anti-NGF mAb like bedinvetmab, intervenes directly in this pathway . Bedinvetmab is a biological protein engineered to recognize and bind circulating canine NGF with high specificity and affinity. By sequestering free NGF, the antibody effectively prevents it from binding to TrkA receptors on nociceptive neurons. This blockade attenuates the entire downstream signaling cascade, reducing both peripheral and central sensitization. Unlike traditional non-steroidal anti-inflammatory drugs (NSAIDs) that inhibit cyclooxygenase (COX) enzymes throughout the body, the mechanism of anti-NGF mAbs is highly focused on a single component of the pain pathway. This specificity is a key advantage, as it minimizes the off-target effects commonly associated with NSAIDs, such as gastrointestinal ulceration, and renal or hepatic toxicity. The introduction of anti-NGF mAbs thus represents a novel therapeutic class for OA pain, offering a targeted molecular approach that directly addresses the mechanisms of pain amplification .
3.2. Clinical Evidence for Efficacy and Safety in Canine OA
The transition of monoclonal antibody therapy from a promising molecular concept to a clinically validated treatment for canine OA is supported by a robust body of evidence from rigorous clinical trials . These studies have been designed to systematically evaluate both the efficacy and safety of anti-NGF mAbs, establishing them as a significant advancement in veterinary pain management. The primary endpoint in these trials is typically the alleviation of pain and the subsequent improvement in the quality of life for dogs suffering from OA.
Numerous clinical studies have demonstrated that anti-NGF therapy is effective in reducing the pain associated with osteoarthritis in dogs . Efficacy is often measured using validated, multi-faceted assessment tools that capture both owner observations and clinician evaluations. These can include instruments like the Canine Brief Pain Inventory (CBPI) and the Liverpool Osteoarthritis in Dogs (LOAD) questionnaire, which quantify pain severity and its interference with daily function. Clinical trials have consistently shown that treatment with bedinvetmab leads to statistically significant improvements in these scores compared to placebo groups. Owners of treated dogs report notable enhancements in their pets’ mobility, activity levels, and overall demeanor. Beyond subjective owner assessments, objective data from preclinical and clinical studies further substantiate the role of NGF in OA pain and the efficacy of its neutralization .
The safety profile of bedinvetmab has also been a focal point of its clinical development program. Laboratory safety evaluations and extensive clinical trial data have been compiled to support its use . Clinical studies focused on safety have concluded that the therapy is generally well-tolerated, with a favorable safety profile that makes it a suitable option for long-term management of a chronic condition . The targeted mechanism of action, which avoids the systemic pathways affected by NSAIDs, contributes to this positive safety assessment. Trials have shown that a single, typically monthly, administration delivers consistent therapeutic effects, a dosing schedule that enhances both compliance and convenience for pet owners . This body of evidence, derived from controlled clinical studies, has been crucial for securing regulatory approval and for providing veterinarians with the confidence to integrate this novel biologic therapy into their treatment protocols for canine OA .
3.3. Pharmacovigilance and Real-World Application (e.g., Bedinvetmab)
Following regulatory approval, the therapeutic role of monoclonal antibodies has been further defined through real-world clinical application and ongoing pharmacovigilance. Bedinvetmab (marketed as Librela) serves as the primary example of this class of therapy in veterinary practice, representing a significant advancement in the management of canine OA pain . Its integration into multimodal pain management plans has provided a valuable option, particularly for dogs that are poor candidates for traditional NSAIDs due to comorbidities (e.g., renal or gastrointestinal disease), intolerance, or insufficient response. The monthly subcutaneous injection schedule offers a practical alternative to daily oral medications, often improving owner compliance and ensuring consistent therapeutic levels.
The post-approval phase of any new therapeutic involves monitoring for adverse drug events (ADEs) in a larger and more diverse patient population than can be studied in controlled clinical trials. This pharmacovigilance is essential for identifying rare or unexpected side effects. Published reports and regulatory agency data have documented some ADEs associated with bedinvetmab administration . A specific area of focus has been the observation of musculoskeletal adverse events. While the overall incidence is low, some cases of worsening lameness or new neurological signs have been reported in dogs receiving the therapy . These observations underscore the importance of thorough veterinary examination and careful patient selection. It is crucial to differentiate disease progression from a potential ADE and to report suspected events to manufacturers and regulatory bodies.
The analysis of real-world case data has provided further insights. For instance, observations have been drawn between the effects seen in dogs and those from human clinical trials of tanezumab, a human anti-NGF mAb, where a small number of patients experienced rapidly progressive OA . While the underlying mechanisms are still being investigated, this highlights the necessity of ongoing monitoring. Despite these reports, the consensus within the veterinary community remains that bedinvetmab has a strong safety profile and that its benefits in alleviating chronic OA pain and improving quality of life for the vast majority of patients are substantial . The real-world experience confirms that while no therapy is without risk, bedinvetmab provides an effective and generally safe targeted treatment, solidifying its place as a cornerstone in the modern, evidence-based management of canine arthritis .
4. Homeopathic Formulations: A Critical Review
Homeopathy is a system of alternative medicine that has been applied to a wide range of conditions in both human and veterinary patients, including chronic diseases like osteoarthritis . Its use in veterinary medicine is often driven by pet owner interest in holistic or “natural” treatment options and a desire to avoid the potential side effects of conventional pharmaceuticals . However, homeopathy operates on principles that are fundamentally different from those of evidence-based pharmacology and physiology. Its proposed mechanisms of action and the nature of its preparations challenge foundational scientific concepts, leading to significant controversy and debate regarding its legitimacy and efficacy. This section provides a critical review of homeopathy as applied to canine OA, examining its core principles, the existing clinical evidence, and the profound methodological challenges inherent in its study.
4.1. Principles of Homeopathy in Veterinary Medicine
The practice of veterinary homeopathy is predicated on two central tenets first articulated by its founder, Samuel Hahnemann, in the late 18th century. The primary principle is similia similibus curentur, Latin for “let like be cured by like.” This “law of similars” posits that a substance capable of producing a specific set of symptoms in a healthy individual can be used to treat similar symptoms in a sick individual. In the context of canine OA, a homeopathic practitioner would select a remedy based on its known ability to cause joint pain or stiffness in a healthy state, under the belief that it will stimulate a healing response in the arthritic patient. This approach involves a detailed “proving” process, where substances are administered to healthy subjects to catalogue the symptoms they produce, which then form the basis of the homeopathic materia medica.
The second, and more scientifically contentious, principle is that of “potentization” through serial dilution and succussion (vigorous shaking). Homeopathic remedies are prepared by systematically diluting a source substance—often a plant, mineral, or animal product—in alcohol or distilled water. A key aspect of this process is that with each step of dilution, the mixture is forcefully shaken, a process known as succussion. Homeopathic theory claims that this action imparts a therapeutic “energy” or “imprint” of the original substance onto the solvent, even as the substance itself becomes progressively scarcer. Many common homeopathic potencies, such as “30C,” represent a dilution factor of 1 part in 10^60. At such extreme dilutions, which far exceed Avogadro’s constant, it is statistically impossible for even a single molecule of the original substance to remain in the final preparation.
This presents a fundamental conflict with the principles of pharmacology and chemistry, which rely on dose-dependent interactions between active molecules and biological receptors. The proposed homeopathic mechanism, often vaguely described as an “energetic” or “vibrational” signal that stimulates the body’s “vital force” to heal itself, is not supported by any known laws of physics or biology. When comparing veterinary drugs with veterinary homeopathy, this schism is central; conventional drugs have demonstrable pharmacokinetic and pharmacodynamic properties, whereas homeopathic products lack a plausible scientific mechanism of action . The practice in veterinary medicine therefore relies on a philosophical framework that is separate from, and often contradictory to, the evidence-based paradigm that governs modern therapeutic development .
4.2. A Critical Review of Clinical Studies on Homeopathy for Pain Management
Despite the widespread use of homeopathy for various veterinary conditions, including the management of pain and arthritis, the body of scientific evidence supporting its efficacy is sparse and of low quality . The evaluation of any therapeutic intervention must be based on well-designed clinical trials that can reliably distinguish a true treatment effect from placebo, natural disease progression, or other confounding factors. When homeopathic treatments for canine OA are subjected to this level of scientific scrutiny, the evidence for a specific therapeutic effect beyond placebo is largely absent.
Systematic reviews, which represent a high level of evidence by aggregating and analyzing the results of multiple studies, have consistently failed to find clear evidence for the benefits of homeopathy . One comprehensive systematic review of veterinary homeopathy found that while some individual studies reported positive outcomes, including some involving dogs, the overall quality of the evidence was poor . Such studies are often plagued by significant methodological flaws, including small sample sizes, lack of adequate blinding, and inappropriate control groups, which severely limit the validity of their conclusions. For instance, a review of randomized trials in veterinary homeopathy that used controls other than placebo was unable to provide any compelling information about a specific treatment effect, highlighting the weakness of the available data .
Some literature syntheses suggest that chronic conditions like arthritis anecdotally present some of the “best results” with homeopathy. However, these same sources critically acknowledge that this perception is not backed by robust data and emphasize the urgent need for clinical trials with a low risk of bias to establish any solid evidence . The existing literature often consists of observational studies, case reports, or trials that do not meet modern standards for evidence-based medicine. For example, a randomized clinical trial on the use of Arnica, a common homeopathic remedy for pain and trauma, was noted in a review, but positive, replicable evidence from such trials remains elusive across the field . The contrast is stark when compared to conventional treatments, where a large body of high-quality clinical trial evidence is a prerequisite for regulatory approval and clinical use . Ultimately, the current scientific literature does not support the claim that homeopathy provides a specific, pharmacologically active benefit for pain management in canine osteoarthritis.
4.3. Analysis of Placebo-Controlled Trials and Methodological Challenges
The gold standard for determining the efficacy of a medical intervention is the randomized, double-blind, placebo-controlled trial (RCT). This design is crucial for minimizing bias and distinguishing a true pharmacological effect from the powerful influence of placebo. When the principles of homeopathy are examined through the lens of the RCT, several profound methodological and conceptual challenges arise that complicate its evaluation and consistently undermine claims of its efficacy.
A primary challenge is the inherent scientific implausibility of the homeopathic mechanism. Because many remedies are diluted to the point of containing no active molecules, there is no testable pharmacological hypothesis. Conventional drug trials are designed around measurable outcomes like blood concentration, receptor occupancy, and dose-response curves. Homeopathy offers no such parameters, making it difficult to design a trial that moves beyond simply asking, “Does it work better than an inert pill?” Furthermore, the tradition of “classical homeopathy,” which involves individualizing remedies for each patient based on a complex constitutional assessment, is fundamentally at odds with the standardized protocols required for RCTs. While some trials have attempted to use standardized combination remedies (e.g., Traumeel), this deviates from the core philosophy of individualized treatment.
The most significant confounder in studies of veterinary homeopathy is the placebo effect, particularly the phenomenon known as the “caregiver placebo effect.” While the animal patient is not susceptible to a belief-based placebo effect, the owner is. An owner who is invested in a treatment and expects a positive outcome is highly likely to perceive an improvement in their pet’s condition, even if none has occurred. They may interpret ambiguous behaviors more optimistically, pay more attention to their pet (which can itself improve well-being), or misattribute natural fluctuations in the symptoms of a chronic disease like arthritis to the remedy . This effect is so powerful that without rigorous blinding of the owner and the assessing veterinarian, it is impossible to draw valid conclusions. Many homeopathic studies lack this essential control. The call for better-designed observational studies in veterinary homeopathy is an attempt to build some form of evidence, but these designs cannot replace the explanatory power of an RCT for demonstrating effectiveness . Ultimately, systematic reviews of high-quality RCTs in both human and veterinary medicine have concluded that homeopathy’s effects are clinically indistinguishable from those of placebo .
5. Comparative Analysis: Evidence, Efficacy, and Clinical Considerations
The management of canine osteoarthritis (OA) presents a clinical crossroads where rapidly advancing biotechnological interventions and long-standing alternative practices converge. Monoclonal antibody (MAT) therapy and homeopathic formulations represent two disparate paradigms in veterinary therapeutics. A comparative analysis of these approaches requires not only an evaluation of their purported efficacy but also a critical deconstruction of their foundational principles, the quality of their supporting evidence, and the complex clinical considerations that influence their application. This section provides a head-to-head comparison of their mechanisms, evaluates the strength of their respective evidence bases according to the principles of evidence-based veterinary medicine (EBVM), and examines the significant confounding role of the placebo effect.
5.1. Head-to-Head Comparison of Mechanisms: Targeted Biology vs. Homeopathic Principles
The fundamental divergence between monoclonal antibody therapy and homeopathy begins at the most basic level: the mechanism of action. MAT is predicated on the principles of modern molecular biology and pharmacology, offering a highly specific, targeted intervention. Homeopathy, in contrast, is based on a set of philosophical tenets established in the 18th century, which operate outside the known principles of chemistry, physics, and physiology.
Monoclonal antibody therapy for canine OA, exemplified by bedinvetmab, functions through a precisely defined and scientifically validated pathway. Its mechanism involves the targeted neutralization of a key pain mediator, Nerve Growth Factor (NGF). Preclinical and clinical studies have unequivocally established that NGF levels are elevated in osteoarthritic joints and play a crucial role in the generation and potentiation of chronic pain signals . Bedinvetmab is a caninized monoclonal antibody designed to bind specifically to and sequester circulating NGF, thereby preventing it from binding to its receptors on nociceptive neurons. This action directly inhibits the transmission of pain signals, reduces neuronal sensitization, and ameliorates the inflammatory cascade associated with NGF, thus providing targeted analgesia. This mechanism is measurable, reproducible, and consistent with the established scientific understanding of pain pathophysiology in OA.
In stark contrast, the proposed mechanism of homeopathy is not grounded in contemporary biological science. It is based on two primary principles: the Law of Similars (“like cures like”), which posits that a substance causing symptoms in a healthy individual can cure similar symptoms in a sick one, and the Principle of Infinitesimals, which involves serial dilution and succussion (vigorous shaking) of a substance. Homeopathic formulations are often diluted to a point where the statistical probability of a single molecule of the original substance remaining in the final preparation is virtually zero. Proponents theorize that the dilution and succussion process imparts a “spirit-like” medicinal force or an “energetic imprint” onto the diluent (typically water or alcohol), which then stimulates the body’s “vital force” to initiate healing. These concepts lack a plausible scientific basis; the idea of “water memory” has been extensively debunked, and the notion of a “vital force” is a metaphysical concept that cannot be empirically measured or tested. Therefore, a direct comparison reveals a dichotomy: MAT offers a specific, molecular-level intervention against a known pathological driver, while homeopathy offers a non-falsifiable, philosophical concept that is incompatible with the principles of pharmacology and chemistry .
5.2. Evaluating the Strength of the Evidence Base: Clinical Trials vs. Anecdotal Reports
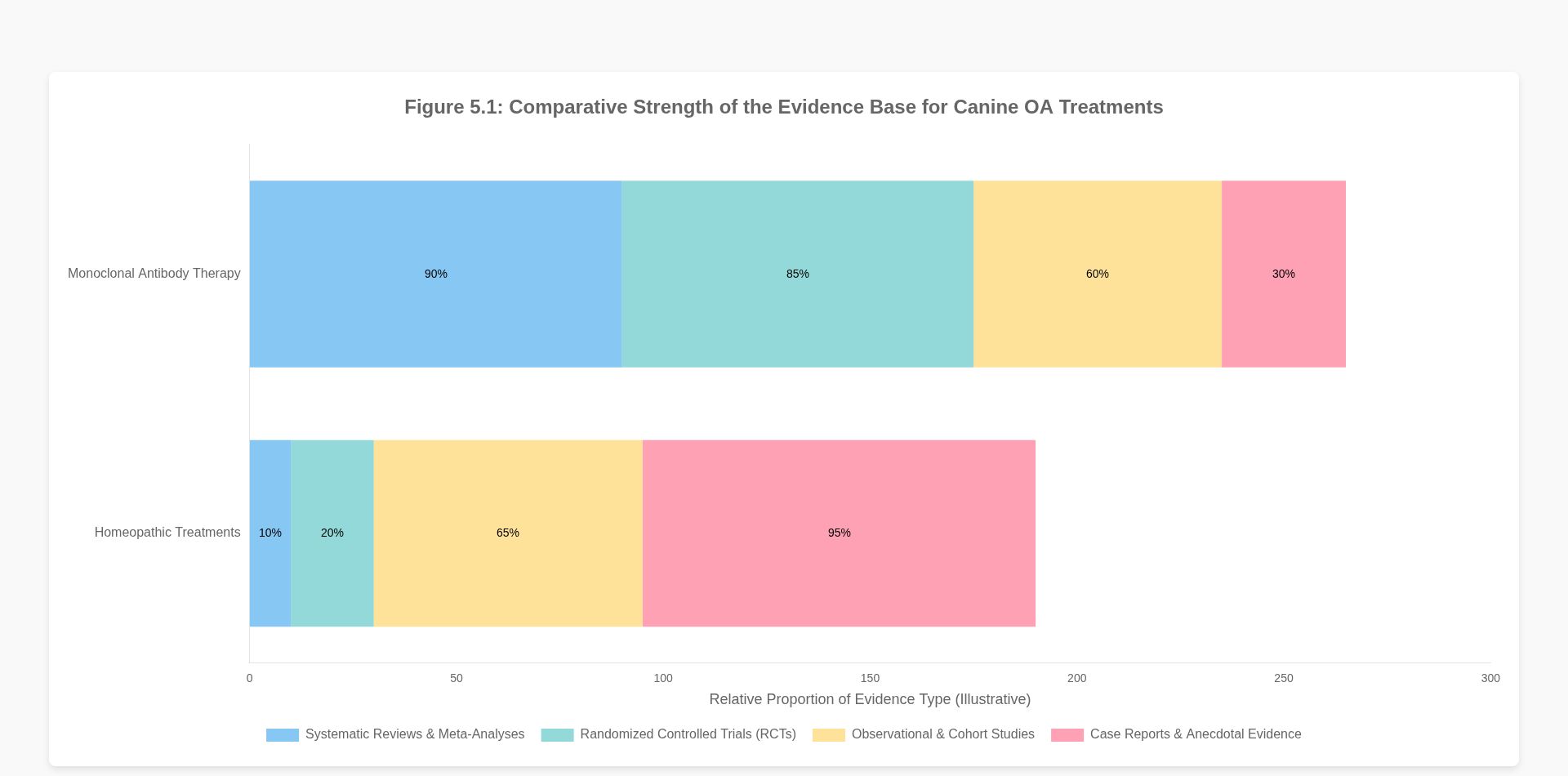
The disparity in scientific plausibility is directly mirrored in the quality and quantity of the evidence base supporting each modality. The framework of evidence-based veterinary medicine (EBVM) provides a structured approach for evaluating this evidence, typically visualized as a hierarchy or “staircase” where systematic reviews of randomized controlled trials (RCTs) represent the highest level of evidence, and expert opinion or anecdotal reports represent the lowest .
The evidence supporting monoclonal antibody therapy for canine OA resides at the upper echelons of this hierarchy. The approval and clinical adoption of agents like bedinvetmab are contingent on a robust portfolio of data derived from rigorous preclinical studies and, most importantly, large-scale, multicenter, randomized, double-blind, placebo-controlled clinical trials . These studies utilize validated owner-completed questionnaires, such as the Canine Brief Pain Inventory (CBPI), and objective measures of lameness to demonstrate a statistically significant improvement in pain scores and quality of life compared to a placebo group . This high-level evidence provides a strong basis for concluding that the observed clinical benefits are a direct result of the drug’s pharmacological action, rather than chance or bias.
Conversely, the evidence base for homeopathy in veterinary medicine is characterized by a reliance on sources traditionally considered low in the evidence hierarchy . While some sources suggest conditions like arthritis may show positive results with homeopathy, they concurrently emphasize the profound need for clinical trials with a low risk of bias to establish any solid evidence . The existing literature is composed largely of anecdotal case reports, observational studies, and clinical trials that are frequently hampered by significant methodological flaws. These flaws include small sample sizes, lack of adequate blinding, absence of a proper control group, and the use of non-validated outcome measures. Systematic reviews of homeopathic trials in veterinary medicine have struggled to find compelling evidence of efficacy. For instance, some reviews conclude that the available evidence is insufficient to provide compelling information for a specific effect beyond placebo . While some individual studies may report positive outcomes for dogs, the overall body of evidence is inconsistent, often contradictory, and fails to meet the standards required by EBVM to prove effectiveness . The push for better-designed observational studies in veterinary homeopathy is an implicit acknowledgment of the current deficit in high-quality evidence .
5.3. The Role of the Placebo Effect and the Caregiver Placebo Phenomenon
No comparative analysis of a therapy with low biological plausibility would be complete without a thorough consideration of the placebo effect. In veterinary medicine, this is a multifaceted phenomenon. While the existence of a direct placebo effect in animals is debated, the “caregiver placebo effect” is a well-documented and powerful confounding variable . It describes the phenomenon where a pet owner’s beliefs and expectations about a treatment influence their perception of the pet’s clinical signs.
When an owner administers a treatment they believe will be effective, several cognitive biases can come into play. They may be more likely to notice and positively interpret subtle improvements in their pet’s mobility or demeanor (confirmation bias), while discounting signs of continued pain. This effect is particularly pronounced in chronic, fluctuating conditions like OA, where good days and bad days occur naturally. An owner may attribute a “good day” to the treatment, reinforcing their belief in its efficacy.
This caregiver placebo effect poses a significant challenge when evaluating therapies like homeopathy, where the perceived effectiveness may be largely or entirely attributable to this phenomenon . Since the owner is typically the one assessing the pet’s pain and quality of life, their subjective reports are highly susceptible to bias if they are not blinded to the treatment being administered. This is precisely why the gold standard for clinical evidence is the randomized, placebo-controlled trial, as this design is specifically intended to neutralize the placebo effect and isolate the true pharmacological effect of the intervention .
Monoclonal antibody therapy has demonstrated its efficacy in such rigorous trials, showing a clear, statistically significant separation from the placebo group. The improvements seen in dogs treated with bedinvetmab are greater than the improvements seen in dogs receiving a placebo injection, confirming a true therapeutic benefit. For homeopathy, however, systematic reviews of randomized placebo-controlled trials have largely failed to demonstrate an effect beyond placebo. The small improvements sometimes reported in both the homeopathy and placebo groups in these studies highlight the strength of the caregiver placebo effect, but they do not provide evidence of an active therapeutic agent. Therefore, when comparing the two, MAT provides an effect that is demonstrably superior to placebo, while the perceived benefits of homeopathy are often indistinguishable from it.
6. Discussion and Implications for Veterinary Practice
The comparative analysis of monoclonal antibody therapy and homeopathic treatments for canine OA reveals a stark divide between an evidence-based, targeted biological intervention and a practice rooted in pre-scientific principles. This chasm has profound implications for veterinary practitioners, who are ethically bound to provide the best possible care based on sound scientific evidence. This section discusses the formulation of an evidence-based treatment hierarchy, strategies for communicating complex information to pet owners, and directions for future research.
6.1. Formulating an Evidence-Based Treatment Hierarchy
In the spirit of evidence-based veterinary medicine (EBVM), clinical decision-making should be guided by a hierarchy of treatments ranked according to the strength of evidence supporting their efficacy and safety . For the management of canine OA pain, such a hierarchy provides a logical and ethical framework for veterinarians.
Tier 1: Foundational, High-Evidence Therapies. At the apex of this hierarchy are interventions with a robust body of evidence from high-quality RCTs and systematic reviews. This tier includes non-steroidal anti-inflammatory drugs (NSAIDs) and, more recently, monoclonal antibody therapies like bedinvetmab. These treatments have demonstrated significant analgesic efficacy, a well-characterized safety profile, and a clear, biologically plausible mechanism of action. They should be considered the cornerstone of pharmacological management for moderate to severe OA pain.
Tier 2: Validated Adjunctive and Supportive Therapies. This second tier includes non-pharmacological interventions that are supported by a solid, though sometimes less standardized, evidence base. This encompasses weight management, controlled low-impact exercise, physical rehabilitation, and nutritional modifications (e.g., supplementation with omega-3 fatty acids). These strategies are crucial for a multimodal approach to OA management and are supported by evidence demonstrating their ability to reduce joint loading, improve muscle support, and enhance overall well-being.
Tier 3: Unproven or Disproven Modalities. The third and lowest tier is reserved for interventions that lack a plausible mechanism of action and have failed to demonstrate efficacy beyond placebo in rigorous scientific testing. Based on the analysis in the preceding sections, homeopathic formulations fall squarely into this category. While generally considered safe due to their extreme dilution, their use for a painful, progressive condition like OA is problematic. Relying on such a modality carries a significant opportunity cost: it can delay the implementation of genuinely effective treatments from Tiers 1 and 2, potentially leading to unnecessary suffering, accelerated disease progression, and a diminished quality of life for the patient. The principles of EBVM dictate that therapies in this tier should not be recommended as a primary or alternative treatment for managing OA pain.
This hierarchical approach, analogous to the “staircase of evidence,” provides a clear, rational pathway for treatment selection, prioritizing patient welfare by ensuring that the most effective and reliable treatments are utilized first .
6.2. Communicating Treatment Options and Evidence to Pet Owners
One of the most significant challenges for veterinarians is navigating conversations with clients who may have strong pre-existing beliefs about “natural” versus “conventional” medicine. Effectively communicating the rationale behind an evidence-based treatment plan is a critical skill that requires empathy, clarity, and a commitment to shared decision-making.
First, it is essential to establish a shared goal: alleviating the pet’s pain and improving its quality of life. By framing the discussion around this common objective, the veterinarian can position themselves as an ally and advocate for the pet.
Second, veterinarians can use simple, accessible analogies to explain the principles of EBVM. For example, the concept of the “staircase of evidence” can be used to visually explain why an RCT for bedinvetmab provides more reliable information than a testimonial about a homeopathic remedy. Explaining that “evidence” is not a monolithic concept but exists in varying levels of quality can help owners understand why veterinarians place more trust in certain types of studies .
Third, addressing the caregiver placebo effect directly but gently is crucial. The veterinarian can acknowledge that owners may indeed see improvement but explain that in scientific studies, similar improvements are often seen in groups receiving a placebo. This helps to depersonalize the conversation and shift the focus from the owner’s perception to the objective, measurable effect of the drug itself.
Fourth, it is vital to address the “natural fallacy”—the belief that natural products are inherently safer or better. Veterinarians can provide examples of potent toxins that are entirely natural (e.g., snake venom, certain plants) to illustrate that “natural” is not synonymous with “safe.” Conversely, they can explain that a therapy like MAT, while high-tech, works by harnessing a natural biological process in a highly targeted way.
Finally, maintaining an open and respectful dialogue is paramount. Dismissing an owner’s beliefs outright is likely to erode trust and may lead them to seek advice from less qualified sources. The goal is not to “win an argument” but to provide the owner with the high-quality information they need to make a truly informed decision that is in their pet’s best interest, guided by the veterinarian’s professional and ethical responsibility.
6.3. Future Research Directions in Canine Arthritis Management
While the current landscape of OA management has been transformed by therapies like MAT, continuous research is essential. Future research should proceed along several parallel tracks.
For monoclonal antibody therapies, long-term pharmacovigilance studies are crucial to continue monitoring for any rare or delayed adverse effects, further solidifying their safety profile. Research into their use in earlier stages of OA, their potential disease-modifying effects, and their efficacy in combination with other modalities (e.g., physical therapy, other analgesics) would be highly valuable. Cost-effectiveness analyses are also needed to help guide both clinical and owner decisions regarding this relatively high-cost therapy.
Regarding homeopathy, the scientific and medical communities have established clear standards for proving the efficacy of any new therapeutic. If proponents of homeopathy wish for it to be accepted within the framework of EBVM, the onus is on them to design and execute large-scale, methodologically impeccable, randomized, double-blind, placebo-controlled trials . These trials must use validated, objective outcome measures and be transparently reported, regardless of the outcome. To date, the homeopathic community has largely failed to produce such evidence for any condition, including canine OA. Without such evidence, homeopathy will rightly remain outside the bounds of evidence-based practice.
More broadly, future research in canine OA should focus on the development of objective biomarkers for pain assessment and disease progression. Such tools would reduce the reliance on subjective owner assessments and provide more accurate measures of therapeutic efficacy for all interventions. Furthermore, the exploration of novel therapeutic targets beyond NGF and the continued development of regenerative medicine strategies hold promise for moving beyond mere symptomatic relief toward true disease modification and joint repair.
7. Conclusion
This comparative analysis of monoclonal antibody therapy and homeopathic treatments for canine osteoarthritis has revealed a fundamental and irreconcilable divergence in scientific principle, evidentiary support, and clinical utility. Monoclonal antibody therapy, represented by bedinvetmab, emerges as a paradigm of modern, evidence-based veterinary medicine. It is founded on a precise and biologically plausible mechanism—the neutralization of Nerve Growth Factor—and its efficacy and safety have been rigorously validated through high-quality, placebo-controlled clinical trials. It represents a significant, targeted, and reliable advancement in the management of chronic OA pain, offering demonstrable improvements in patient quality of life.
In contrast, homeopathic formulations for canine OA lack both a scientifically plausible mechanism of action and a robust body of supporting evidence. The practice is predicated on 18th-century philosophical tenets that are incongruent with foundational principles of modern chemistry, pharmacology, and physiology. The evidence base is dominated by low-quality studies, anecdotal reports, and trials fraught with methodological weaknesses. In rigorous, placebo-controlled settings, homeopathy has not been shown to provide a clinical effect superior to that of placebo, indicating that any perceived benefits are likely attributable to the powerful influence of the caregiver placebo effect, natural disease fluctuation, or other confounding variables.
For the veterinary practitioner, the implications of this analysis are clear. The ethical and professional obligation to prioritize animal welfare necessitates adherence to the principles of evidence-based veterinary medicine. This involves constructing a treatment hierarchy that places therapies with proven efficacy and safety, such as monoclonal antibodies, at the forefront of clinical management. Unproven modalities like homeopathy, which lack evidence of effect beyond placebo, should not be recommended as they risk prolonging patient suffering by delaying or supplanting effective, evidence-based care. While respectful and open communication with pet owners is essential, the veterinarian’s ultimate responsibility is to serve as a scientific advocate for the patient. By embracing evidence-based principles, the veterinary profession can ensure it provides the highest standard of care, effectively alleviating the pain of canine osteoarthritis and enhancing the welfare of the animals entrusted to its charge.
References
APA
Dodds, W… (2025). One Health: Introduction to Integrative Veterinary Medicine. Veterinary Clinics: Small Animal Practice. https://www.vetsmall.theclinics.com/article/S0195-5616(25)00093-2/abstract
Ali, A… (2021). Pathophysiology of osteoarthritis and Current Treatment. Zagazig Veterinary Journal. https://journals.ekb.eg/article_160267.html
Onbysh, T., Sampiev, A., & Semenenko, M… (2024). OSTEOARTHRITISES IN DOGS: ETIOPATHOGENESIS AND MODERN APPROACHES TO THERAPY. http://vfvrn.ru/netcat_files/6/2/VFV_2024_3_28_1__0.pdf#page=62
Panizzi, L… (2024). … with induced carpal osteoarthritis: a thesis presented in partial fulfilment of the requirements for the degree of Doctor of Philosophy in Veterinary Science at Massey …. mro.massey.ac.nz. https://mro.massey.ac.nz/handle/10179/71430
Wang, A., Aviña, A., Liu, Y., & Kao, H… (2025). Stromal vascular fraction in canine osteoarthritis: advantages, applications, and insights for veterinary practitioners. https://www.frontiersin.org/journals/veterinary-science/articles/10.3389/fvets.2025.1586629/abstract
Tomé, I., Alves-Pimenta, S., Sargo, R., & Pereira…, J… (2023). Mechanical osteoarthritis of the hip in a one medicine concept: a narrative review. https://link.springer.com/article/10.1186/s12917-023-03777-z
Szponder, T., Latalski, M., Danielewicz, A., & Krać…, K… (2022). Osteoarthritis: pathogenesis, animal models, and new regenerative therapies. https://www.mdpi.com/2077-0383/12/1/5
Alves, J… (2021). Evaluation of the efficacy of four intra-articular therapeutic protocols for the control and treatment of osteoarthritis in a Canis familiaris model. search.proquest.com. https://search.proquest.com/openview/8d14c7ef150577de2b420fab80278c66/1?pq-origsite=gscholar&cbl=2026366&diss=y
Block, G… (2024). Evidence‐based veterinary medicine—potential, practice, and pitfalls. Journal of Veterinary Internal Medicine. https://onlinelibrary.wiley.com/doi/abs/10.1111/jvim.17239
Reid, J., Gildea, E., Davies, V., & Thompson…, J… (2024). Measuring the effect of the anti-nerve growth factor antibodies bedinvetmab and frunevetmab on quality of life in dogs and cats with osteoarthritis using a validated …. https://www.frontiersin.org/journals/veterinary-science/articles/10.3389/fvets.2024.1395360/full
Banu, S., Sharun, K., & Emmanuel…, R… (2025). Stem Cell Exosomes for Osteoarthritis in Veterinary Medicine. https://onlinelibrary.wiley.com/doi/abs/10.1155/sci/4888569
Kurhaluk, N. & Tkaczenko, H… (2025). Recent Issues in the Development and Application of Targeted Therapies with Respect to Individual Animal Variability. Animals. https://www.mdpi.com/2076-2615/15/3/444
Werts, A., Reece, D., Simon, T., & Cole, P… (2024). Re: Re: Laboratory safety evaluation of bedinvetmab, a canine anti-nerve growth factor monoclonal antibody, in dogs. The Veterinary Journal. https://www.sciencedirect.com/science/article/pii/S109002332400114X
Wang, J., Zhou, X., Elazab, S., Huang, J., & Hsu, W… (2025). Current Review of Monoclonal Antibody Therapeutics in Small Animal Medicine. Animals. https://www.mdpi.com/2076-2615/15/4/472
Arnade, H., Ivie, I., & Gordon…, J… (2025). Three-dimensional tissue culture supports the structure and function of equine synovial explants over 4 days. https://avmajournals.avma.org/view/journals/ajvr/aop/ajvr.24.11.0357/ajvr.24.11.0357.xml
Epstein, M., Frye, C., & Campoy, L… (2025). The prevention and management of pain in dogs. https://onlinelibrary.wiley.com/doi/abs/10.1002/9781394251452.ch6
Lane, D. & Bailey, K… (2025). Multimodal Approach to Canine Arthritis. Canine Sports Medicine and Rehabilitation. https://onlinelibrary.wiley.com/doi/abs/10.1002/9781394251452.ch9
Amatucci, H. & Medeiros…, N. D… (2024). Use of Homeopathy in Chronic Diseases of Veterinary Interest: a Literature Synthesis. https://www.highdilution.org/index.php/ijhdr/article/view/1473
Lees, P., Pelligand, L., Whiting, M., & Chambers…, D… (2017). Comparison of veterinary drugs and veterinary homeopathy: part 1. https://bvajournals.onlinelibrary.wiley.com/doi/abs/10.1136/vr.104278
Bergh, A., Lund, I., Boström, A., Hyytiäinen, H., & Asplund, K… (2021). A systematic review of complementary and alternative veterinary medicine:“Miscellaneous therapies”. Animals. https://www.mdpi.com/2076-2615/11/12/3356
Hershey, B… (2025). Integrative Veterinary Oncology: Part II–Homeopathy, Acupuncture, Manual Therapies, Oxygen Therapies, Energy Work and Phototherapies. Veterinary Clinics: Small Animal Practice. https://www.vetsmall.theclinics.com/article/S0195-5616(25)00095-6/abstract
Bez, I., Paula, G. D., & Revers…, N… (2024). The use of homeopathy in veterinary medicine: a systematic review. https://pdfs.semanticscholar.org/9c68/4df0ea53938c7f61304f3e579fb232f38e4c.pdf
Mathie, R. & Clausen, J… (2015). Veterinary homeopathy: systematic review of medical conditions studied by randomised trials controlled by other than placebo. BMC Veterinary Research. https://link.springer.com/article/10.1186/s12917-015-0542-2
Lees, P., Pelligand, L., Whiting, M., & Chambers…, D… (2017). Comparison of veterinary drugs and veterinary homeopathy: part 2. https://bvajournals.onlinelibrary.wiley.com/doi/abs/10.1136/vr.104279
Weiermayer, P., Frass, M., & Fibert…, P… (2023). Recommendations for designing, conducting and reporting clinical observational studies in homeopathic veterinary medicine. https://www.thieme-connect.com/products/all/doi/10.1055/s-0043-1760845
Arlt, S. & Heuwieser, W… (2016). The staircase of evidence–a new metaphor displaying the core principles of evidence-based veterinary medicine. Veterinary Evidence. https://veterinaryevidence.org/index.php/ve/article/view/18
Mills, D., Reiss, M., & Campbell, M… (2025). Evidence-based veterinary medicine at 20–a commentary on historical, philosophical, practical and ethical aspects. Veterinary evidence. https://discovery.ucl.ac.uk/id/eprint/10212342/